





Initial diagnosis:
Stage IIB tumor identified in a routine mammogram
Stage IIB tumor identified in a routine mammogram
- Mass identified in a routine mammogram, and excision biopsy performed
- Diagnosis:
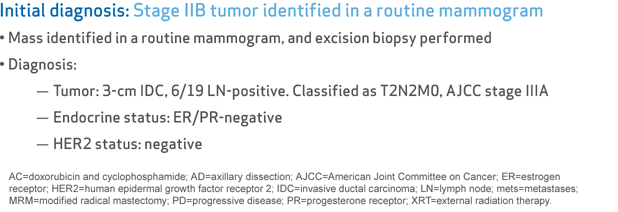
- Tumor: 3-cm IDC, 6/19 LN-positive. Classified as T2N2M0, AJCC stage IIIA
- Endocrine status: ER/PR-negative
- HER2 status: negative
AC=doxorubicin and cyclophosphamide; AD=axillary dissection; AJCC=American Joint Committee on Cancer; ER=estrogen receptor; HER2=human epidermal growth factor receptor 2; IDC=invasive ductal carcinoma; LN=lymph node; mets=metastases; MRM=modified radical mastectomy; PD=progressive disease; PR=progesterone receptor; XRT=external radiation therapy.
Undergoes surgery and adjuvant treatment
- Left MRM and AD performed
- Adjuvant therapy (AC-based chemotherapy) followed by XRT to the left chest wall
AC=doxorubicin and cyclophosphamide; AD=axillary dissection; mets=metastases; MRM=modified radical mastectomy; PD=progressive disease; XRT=external radiation therapy.
Metastatic disease relapse
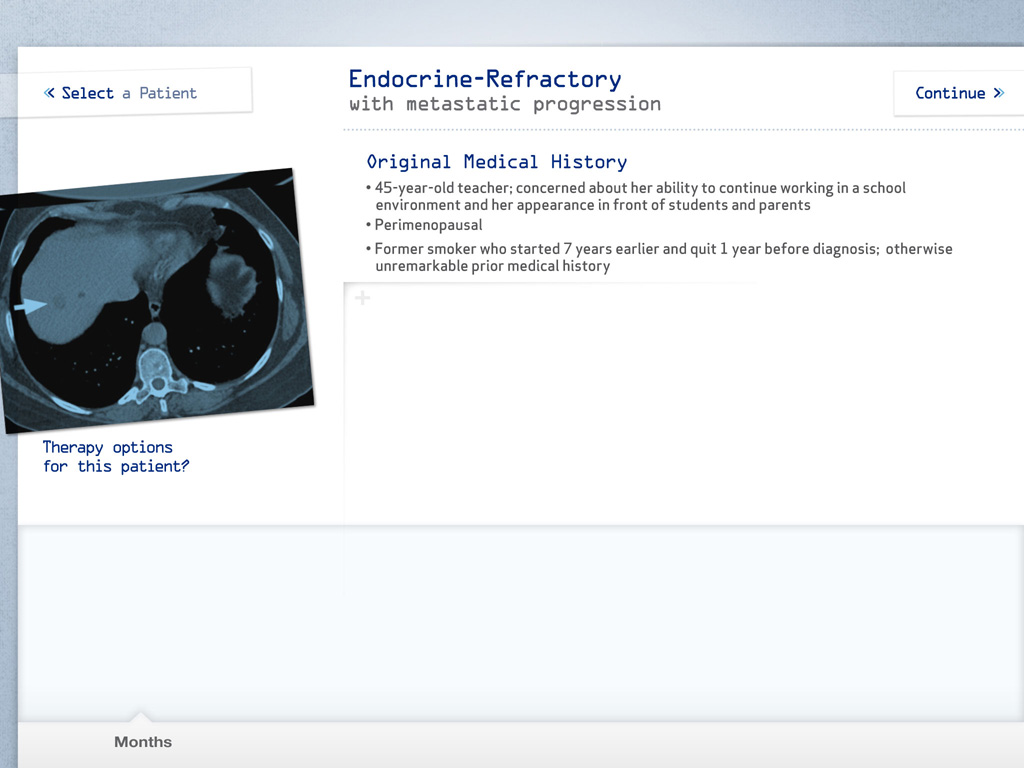
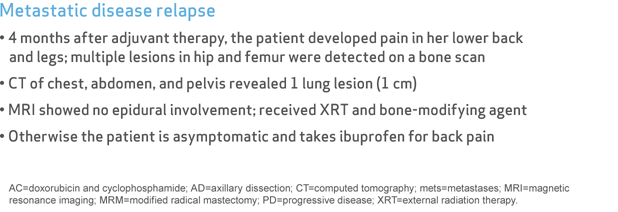
- 4 months after adjuvant therapy, the patient developed pain in her lower back and legs; multiple lesions in hip and femur were detected on a bone scan
- CT of chest, abdomen, and pelvis revealed 1 lung lesion (1 cm)
- MRI showed no epidural involvement; received XRT and bone-modifying agent
- Otherwise the patient is asymptomatic and takes ibuprofen for back pain
AC=doxorubicin and cyclophosphamide; AD=axillary dissection; CT=computed tomography; mets=metastases; MRI=magnetic resonance imaging; MRM=modified radical mastectomy; PD=progressive disease; XRT=external radiation therapy.
Initial diagnosis:
Stage IIB tumor detected in self-examination
Stage IIB tumor detected in self-examination
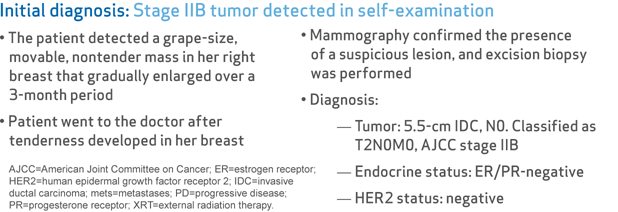
- The patient detected a grape-size, movable, nontender mass in her right breast that gradually enlarged over a 3-month period
- Patient went to the doctor after tenderness developed in her breast
- Mammography confirmed the presence of a suspicious lesion, and excision biopsy was performed
- Diagnosis:
- Tumor: 5.5-cm IDC, N0. Classified as T2N0M0, AJCC stage IIB
- Endocrine status: ER/PR-negative
- HER2 status: negative
AJCC=American Joint Committee on Cancer; ER=estrogen receptor; HER2=human epidermal growth factor receptor 2; IDC=invasive ductal carcinoma; mets=metastases; PD=progressive disease; PR=progesterone receptor; XRT=external radiation therapy.
Declines standard treatment for locally advanced disease
- Originally diagnosed 2 years ago with resectable disease
- The patient refused standard treatment and sought alternative therapy
mets=metastases; PD=progressive disease; XRT=external radiation therapy.
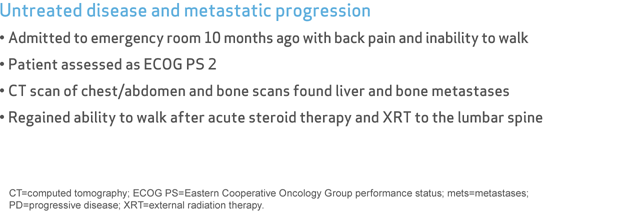
Untreated disease and metastatic progression
- Admitted to emergency room 10 months ago with back pain and inability to walk
- Patient assessed as ECOG PS 2
- CT scan of chest/abdomen and bone scans found liver and bone metastases
- Regained ability to walk after acute steroid therapy and XRT to the lumbar spine
CT=computed tomography; ECOG PS=Eastern Cooperative Oncology Group performance status; mets=metastases; PD=progressive disease; XRT=external radiation therapy.
Combination chemotherapy to address residual metastases
- History of cardiomyopathy with pregnancy; echocardiogram revealed decreased ejection fraction
- Platinum-based therapy in first line was well tolerated
- Patient achieved a partial response
- Disease progressed after 7 months of treatment
Initial diagnosis:
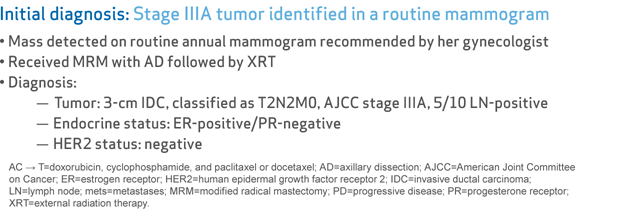
Stage IIIA tumor identified in a routine mammogram
Stage IIIA tumor identified in a routine mammogram
- Mass detected on routine annual mammogram recommended by her gynecologist
- Received MRM with AD followed by XRT
- Diagnosis:
- Tumor: 3-cm IDC, classified as T2N2M0, AJCC stage IIIA, 5/10 LN-positive
- Endocrine status: ER-positive/PR-negative
- HER2 status: negative
AC → T=doxorubicin, cyclophosphamide, and paclitaxel or docetaxel; AD=axillary dissection; AJCC=American Joint Committee on Cancer; ER=estrogen receptor; HER2=human epidermal growth factor receptor 2; IDC=invasive ductal carcinoma; LN=lymph node; mets=metastases; MRM=modified radical mastectomy; PD=progressive disease; PR=progesterone receptor; XRT=external radiation therapy.
Anthracycline-based adjuvant treatment
- Adjuvant AC → T followed by tamoxifen
- Therapy was well tolerated; patient became amenorrheic during chemotherapy
AC → T=doxorubicin, cyclophosphamide, and paclitaxel or docetaxel; mets=metastases; MRM=modified radical mastectomy; PD=progressive disease; XRT=external radiation therapy.
Metastatic relapse after long-term hormonal therapy
- After 3 years of tamoxifen therapy, patient complained of pain; bone scan revealed metastases
- Biopsy of bone metastasis confirmed ER-positive, HER2-negative disease
- Patient remained amenorrheic; estradiol levels confirmed she is postmenopausal
AC → T=doxorubicin, cyclophosphamide, and paclitaxel or docetaxel; ER=estrogen receptor; HER2=human epidermal growth factor receptor 2; mets=metastases; MRM=modified radical mastectomy; PD=progressive disease; PR=progesterone receptor; XRT=external radiation therapy.
Repeated progression
- Given anastrozole, bone-modifying agent, and XRT for bone metastases; pain subsided initially, but progression was seen after 6 months
- The patient was treated with exemestane + everolimus
- Further progression of bone metastases occurred at 5 months; CT scan revealed new liver metastases
- Given capecitabine + ixabepilone; progressed again after 5.5 months
AC → T=doxorubicin, cyclophosphamide, and paclitaxel or docetaxel; CT=computed tomography; mets=metastases; MRM=modified radical mastectomy; PD=progressive disease; XRT=external radiation therapy.
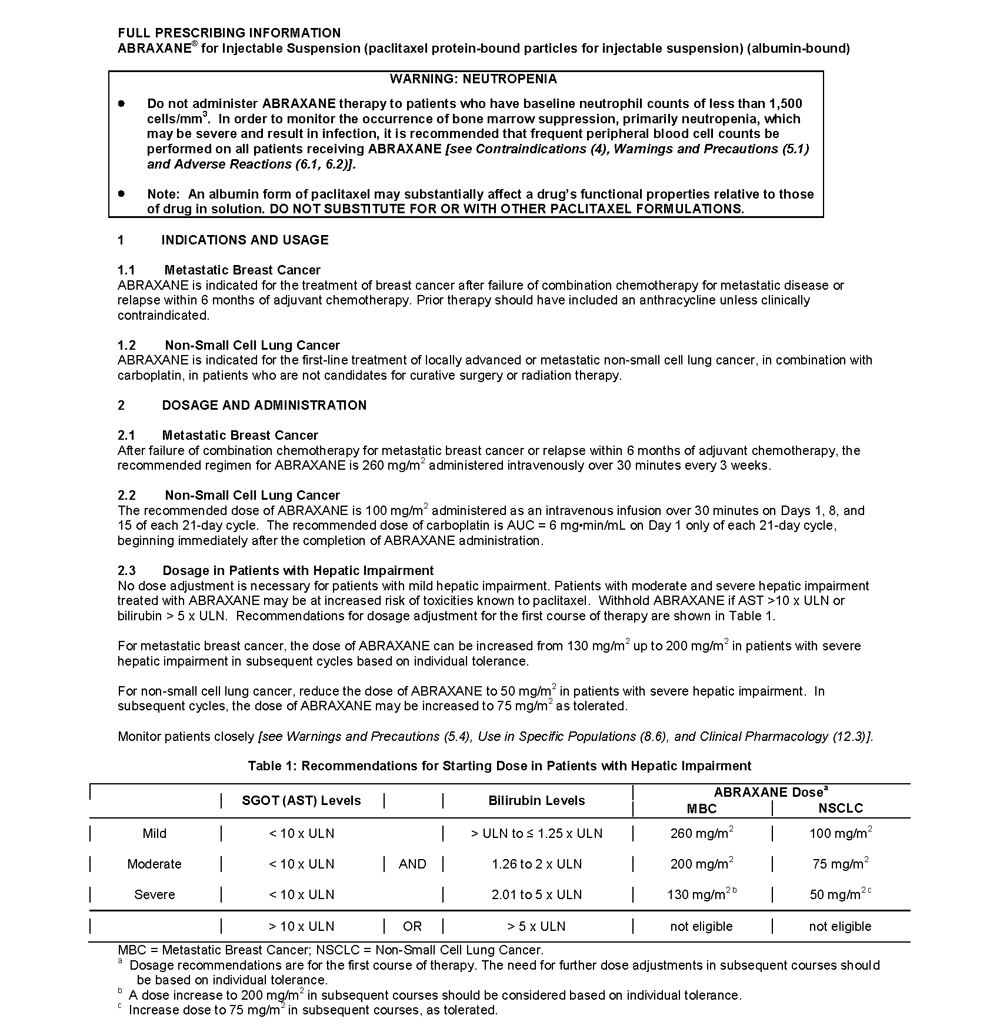
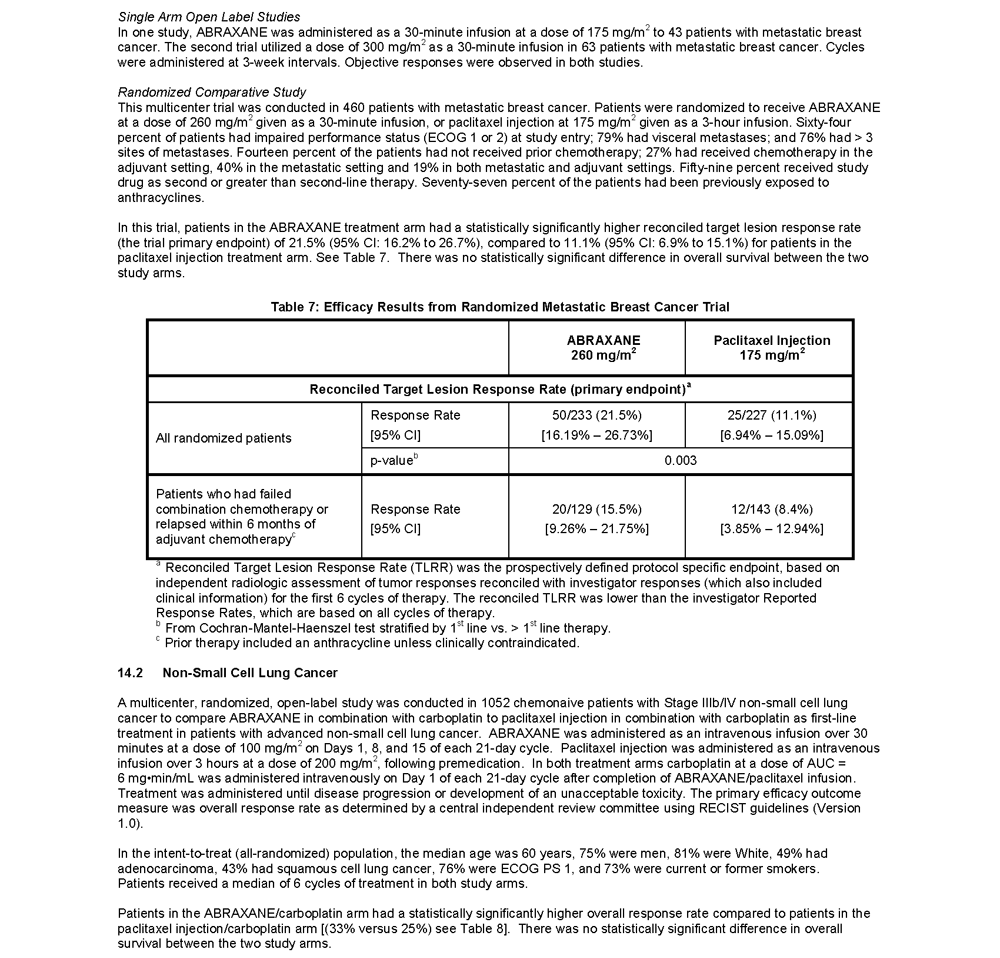
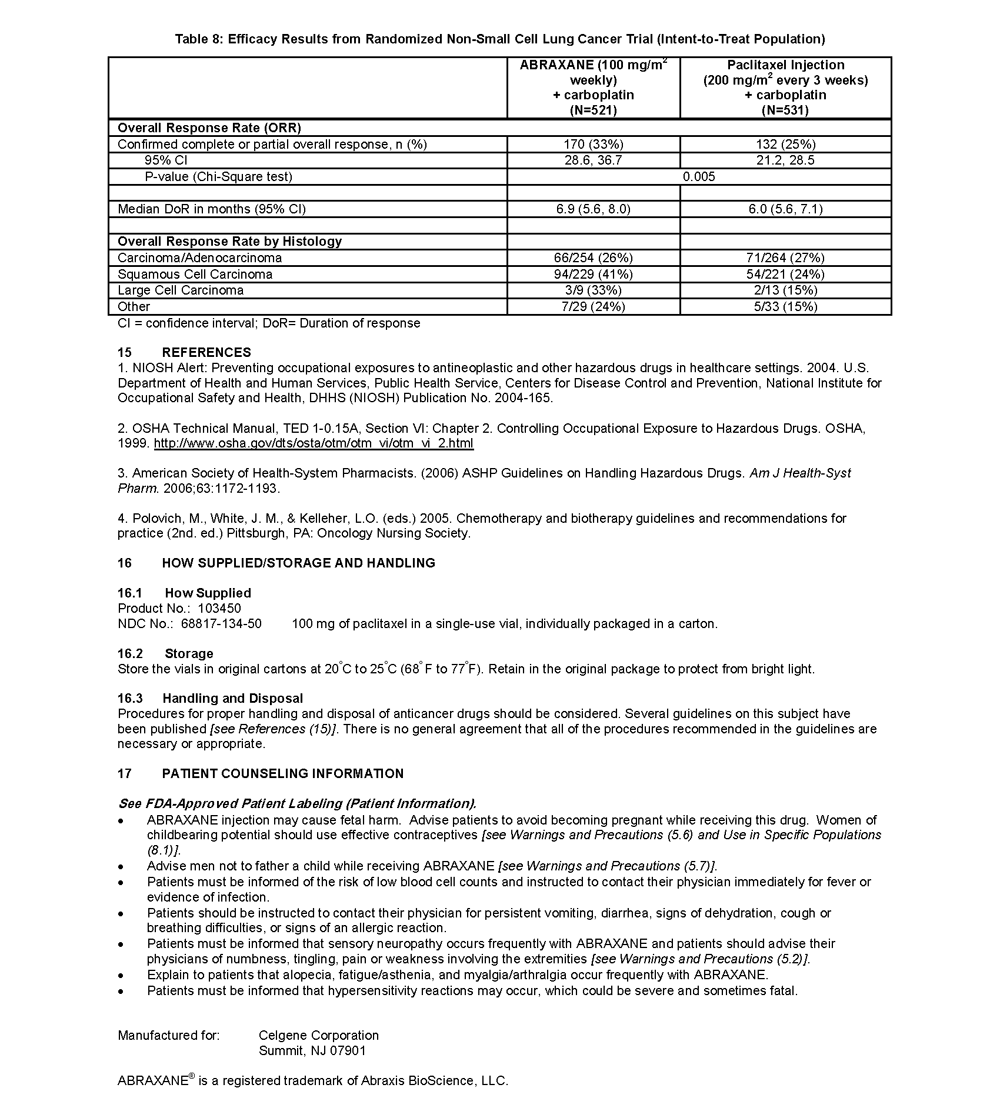
ABRAXANE® for Injectable Suspension (paclitaxel protein-bound particles for injectable suspension) (albumin-bound) is indicated for the treatment of breast cancer after failure of combination chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy. Prior therapy should have included an anthracycline unless clinically contraindicated.
Important Safety Information
WARNING - NEUTROPENIA
- Do not administer ABRAXANE therapy to patients who have baseline neutrophil counts of less than 1,500 cells/mm³. In order to monitor the occurrence of bone marrow suppression, primarily neutropenia, which may be severe and result in infection, it is recommended that frequent peripheral blood cell counts be performed on all patients receiving ABRAXANE
- Note: An albumin form of paclitaxel may substantially affect a drug's functional properties relative to those of drug in solution. DO NOT SUBSTITUTE FOR OR WITH OTHER PACLITAXEL FORMULATIONS
CONTRAINDICATIONS
Neutrophil Counts
- ABRAXANE should not be used in patients who have baseline neutrophil counts of < 1,500 cells/mm³
- Patients who experience a severe hypersensitivity reaction to ABRAXANE should not be rechallenged with the drug
WARNINGS AND PRECAUTIONS
Hematologic Effects
- Bone marrow suppression (primarily neutropenia) is dose-dependent and a dose-limiting toxicity of ABRAXANE. In a clinical study, Grade 3-4 neutropenia occurred in 34% of patients with metastatic breast cancer (MBC)
- Monitor for myelotoxicity by performing complete blood cell counts frequently, including prior to dosing on Day 1 for MBC
- Do not administer ABRAXANE to patients with baseline absolute neutrophil counts (ANC) of less than 1,500 cells/mm³
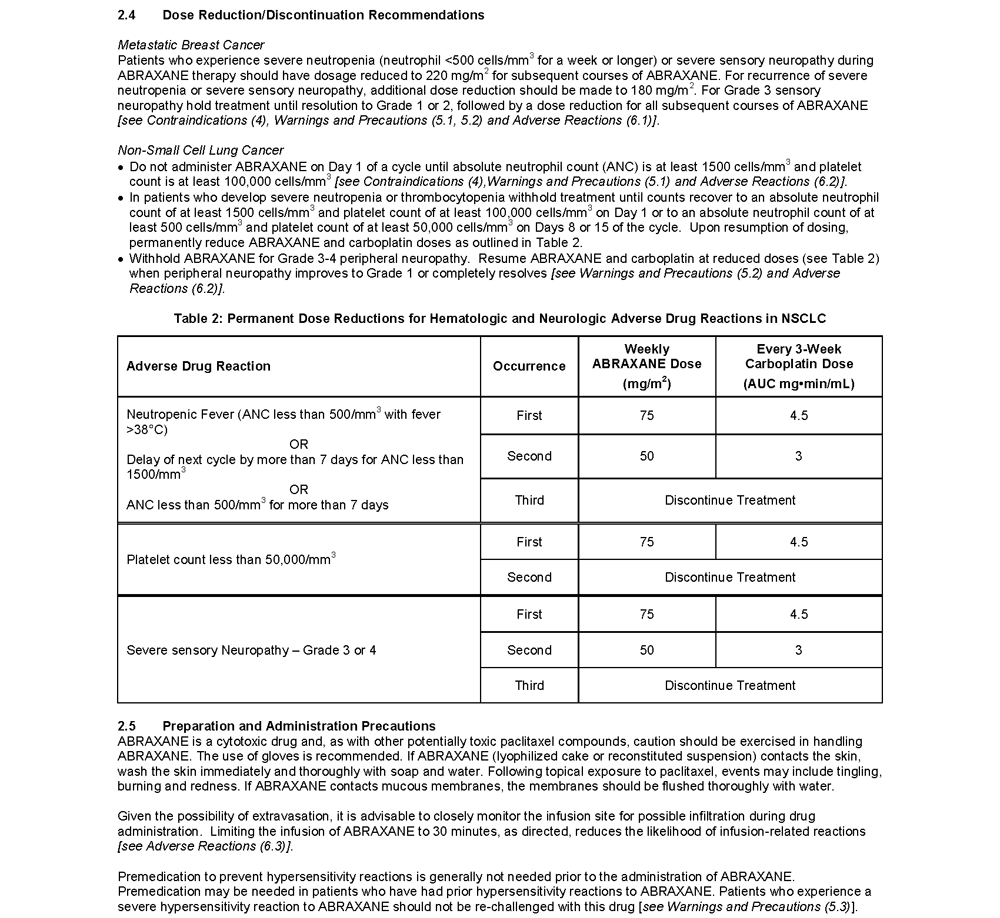
- In the case of severe neutropenia (<500 cells/mm³ for 7 days or more) during a course of ABRAXANE therapy, reduce the dose of ABRAXANE in subsequent courses in patients with MBC
- In patients with MBC, resume treatment with every-3-week cycles of ABRAXANE after ANC recovers to a level >1,500 cells/mm³ and platelets recover to >100,000 cells/mm³
- Sensory neuropathy is dose- and schedule-dependent
- The occurrence of Grade 1 or 2 sensory neuropathy does not generally require dose modification
- If ≥ Grade 3 sensory neuropathy develops, treatment should be withheld until resolution to Grade 1 or 2 for MBC followed by a dose reduction for all subsequent courses of ABRAXANE
- Severe and sometimes fatal hypersensitivity reactions, including anaphylactic reactions, have been reported
- Patients who experience a severe hypersensitivity reaction to ABRAXANE should not be re-challenged with this drug
- Because the exposure and toxicity of paclitaxel can be increased with hepatic impairment, administration of ABRAXANE in patients with hepatic impairment should be performed with caution
- The starting dose should be reduced for patients with moderate or severe hepatic impairment
- ABRAXANE contains albumin (human), a derivative of human blood
- ABRAXANE can cause fetal harm when administered to a pregnant woman
- If this drug is used during pregnancy, or if the patient becomes pregnant while receiving this drug, the patient should be apprised of the potential hazard to the fetus
- Women of childbearing potential should be advised to avoid becoming pregnant while receiving ABRAXANE
- Men should be advised not to father a child while receiving ABRAXANE
ADVERSE REACTIONS
Randomized Metastatic Breast Cancer (MBC) Study
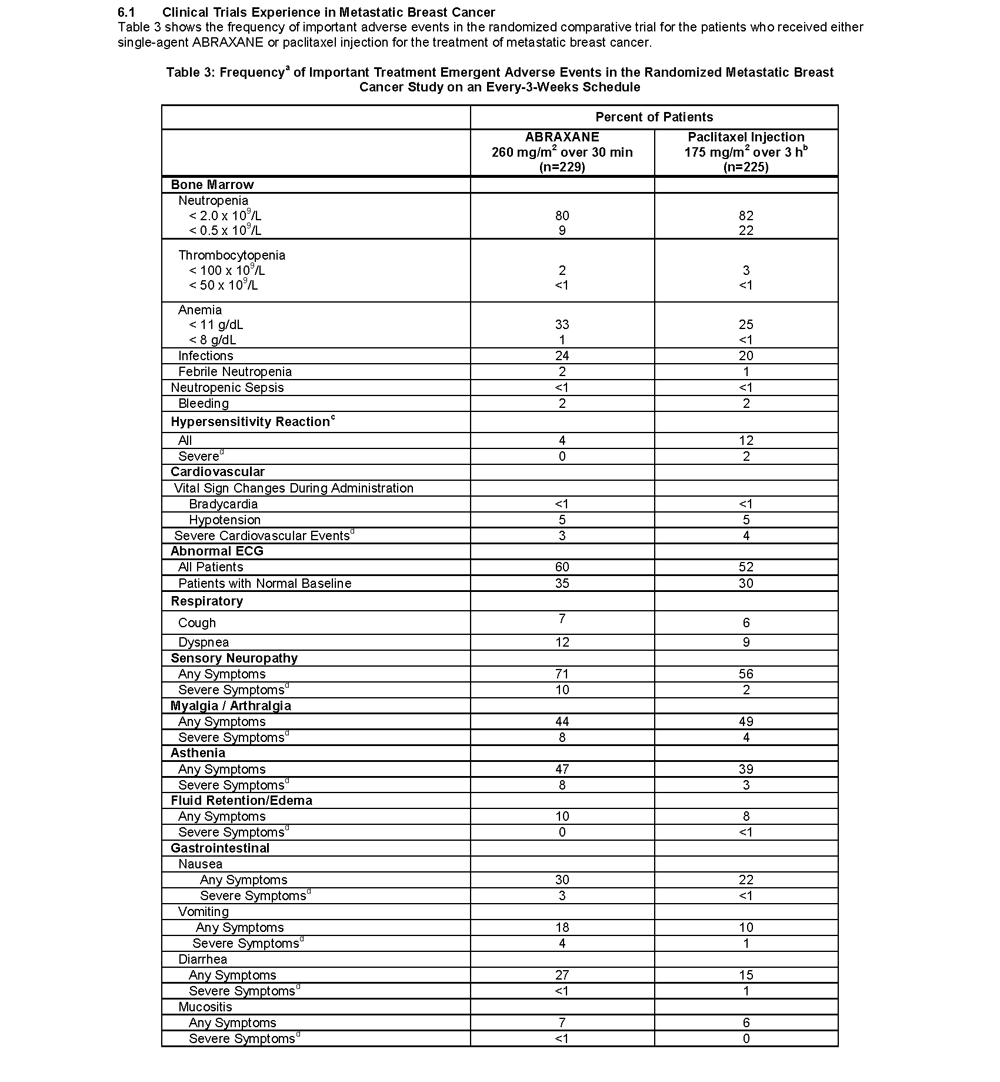
- The most common adverse reactions (≥20%) with single-agent use of ABRAXANE vs. Paclitaxel injection in the MBC study were alopecia (90%, 94%), neutropenia (all cases 80%,82%; severe 9%,22%), sensory neuropathy (any symptoms 71%, 56%; severe 10%, 2%), abnormal ECG (all patients 60%, 52%; patients with normal baseline 35%, 30%), fatigue/asthenia (any 47%, 39%; severe 8%, 3%), myalgia/arthralgia (any 44%, 49%; severe 8%, 4%), AST elevation (any 39%, 32%), alkaline phosphatase elevation (any 36%, 31%), anemia (all cases 33%, 25%; severe 1%, <1%), nausea (any 30%, 22%; severe 3%, <1%), diarrhea (any 27%, 15%; severe <1%, 1%) and infections (24%, 20%), respectively
- Sensory neuropathy was the cause of ABRAXANE discontinuation in 7/229 (3%) patients
- Other adverse reactions of note with the use of ABRAXANE vs. Paclitaxel injection included vomiting (any 18%,10%; severe 4%, 1%), fluid retention (any 10%,8%; severe 0%,<1%); mucositis (any 7%, 6%; severe <1%, 0%), hepatic dysfunction (elevations in bilirubin 7%, 7%), hypersensitivity reactions (any 4%,12%; severe 0%, 2%), thrombocytopenia (any 2%, 3%; severe <1%, <1%), neutropenic sepsis (<1%, <1%), and injection site reactions (<1%, 1%), respectively. Dehydration and pyrexia were also reported
- Renal dysfunction (any 11%, severe 1%) were reported in patients treated with ABRAXANE (n = 229)
- In all ABRAXANE treated patients (n=366) ocular/visual disturbances were reported (any 13%; severe 1%)
- Severe cardiovascular events possibly related to single-agent ABRAXANE occurred in approximately 3% of patients and included cardiac ischemia/infarction, chest pain, cardiac arrest, supraventricular tachycardia, edema, thrombosis, pulmonary thromboembolism, pulmonary emboli, and hypertension
- Cases of cerebrovascular attacks (strokes) and transient ischemic attacks have been reported
- Severe and sometimes fatal hypersensitivity reactions have been reported with ABRAXANE. The use of ABRAXANE in patients previously exhibiting hypersensitivity to paclitaxel injection or to human albumin has not been studied
- There have been reports of congestive heart failure and left ventricular dysfunction with ABRAXANE, primarily among individuals with underlying cardiac history or prior exposure to cardiotoxic drugs
- There have been reports of extravasation of ABRAXANE. Given the possibility of extravasation, it is advisable to monitor closely the ABRAXANE infusion site for possible infiltration during drug administration
DRUG INTERACTIONS
- Caution should be exercised when administering ABRAXANE concomitantly with medicines known to inhibit or induce either CYP2C8 or CYP3A4
USE IN SPECIFIC POPULATIONS
Nursing Mothers
- It is not known whether paclitaxel is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants, a decision should be made to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother
- The safety and effectiveness of ABRAXANE in pediatric patients have not been evaluated
- No toxicities occurred notably more frequently among patients ≥65 years of age who received ABRAXANE for MBC
- The use of ABRAXANE has not been studied in patients with renal impairment
DOSAGE AND ADMINISTRATION
- Dose adjustment is recommended for patients with moderate and severe hepatic impairment and patients who experience severe neutropenia or severe sensory neuropathy during treatment with ABRAXANE
- Withhold ABRAXANE if AST >10 x ULN or bilirubin >5 x ULN
- Dose reductions or discontinuation may be needed based on severe hematologic or neurologic toxicities
- Monitor patients closely